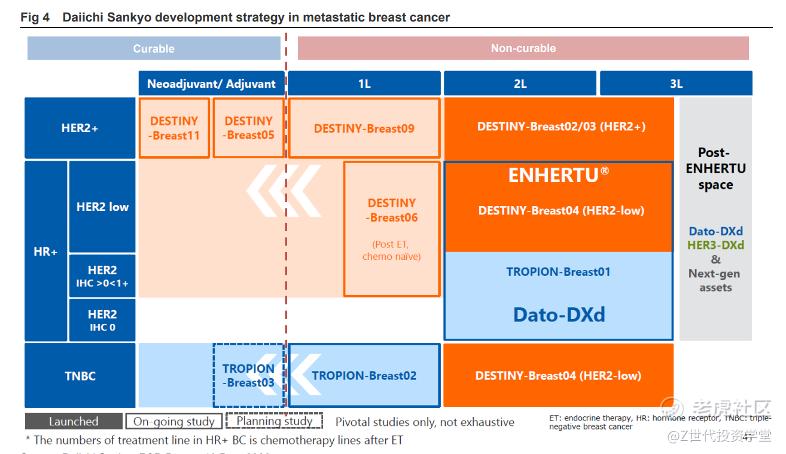
Initial Report(part2): Daiichi Sankyo (4568.T), 67% 5-yr Potential Upside (VIP GC, 谭天禹)
The ADC market is becoming crowded with more MNCs exploring the candidates. Different MNCs adopt different strategies:
AstraZeneca: AstraZeneca strategically acquired Spirogen, a pioneer in PBD-based ADC technology, allowing them to enter the ADC field. They also invested in ADC Therapeutics and collaborated with Daiichi Sankyo, further expanding their presence in the ADC market.
AbbVie and Gilead: These companies secured their positions in the ADC field through significant acquisitions. By acquiring companies with established ADC technologies, products, or pipelines, AbbVie and Gilead gained a foothold in the ADC market.
Roche: Roche represents a risk-averse strategy by focusing on collaborations rather than developing their own ADC platform. They partnered with ImmunoGen to obtain the successful ADC product Kadcyla and collaborated with Seagen to launch Polivy, both of which have shown positive sales performance.
Novartis: Novartis followed a similar strategy as a latecomer to the ADC field. They established collaborations, including a collaboration with Daiichi Sankyo, to access promising ADC technologies or products and enter the market.
Merck: Like Novartis, Merck adopted a strategy of collaboration to enter the ADC field. They collaborated with Seagen to develop and commercialize ADCs.
Pfizer: Pfizer initially entered the ADC field through the acquisition of Wyeth, which included the world's first ADC, Mylotarg. However, they shifted their focus away from ADCs for a period. Recently, with the success of ADC Therapeutics' products, Pfizer regained interest in the ADC field and is reportedly in preliminary negotiations to acquire Seagen, which could significantly boost its presence in the ADC market.
Daiichi Sankyo: Daiichi Sankyo has strategically collaborated with multiple pharmaceutical companies, including AstraZeneca, Novartis, and Pfizer, to develop and commercialize ADCs. These collaborations have allowed Daiichi Sankyo to leverage its expertise in ADC technology and expand its presence in the ADC market.
Investment Thesis
ADC technology spreadheads to shape the treatment of cancer
ADC technology is shaping the treatment of cancer by increasing the efficacy of anticancer medications, minimizing systemic drug distribution, and providing novel therapeutic alternatives for diseases with poor prognoses and few treatment options
Increased Efficacy
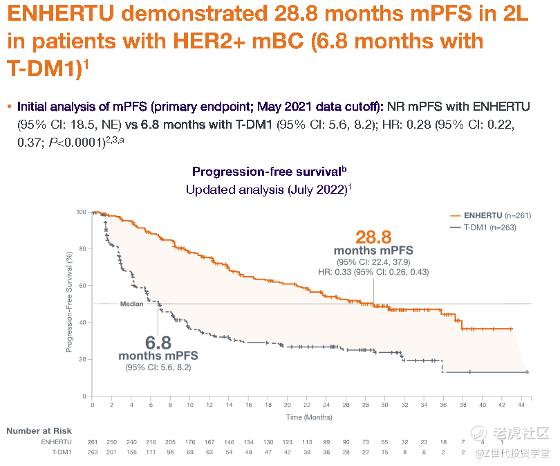
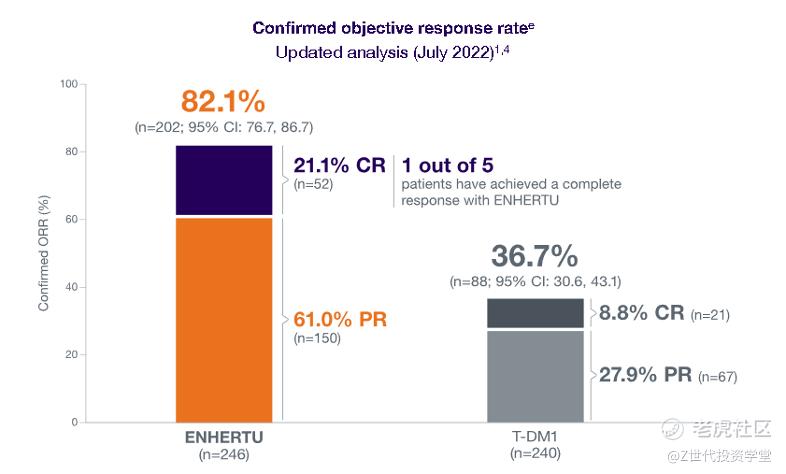
ADCs have shown promising results in clinical trials. For example, in a phase 3 trial, Enhertu, an ADC developed by AstraZeneca and Daiichi Sankyo, demonstrated a statistically significant improvement in progression-free survival compared to standard-of-care chemotherapy in patients with HER2-positive metastatic breast cancer. The median progression-free survival was 28.8 months in the Enhertu group compared to 5.6 months in the chemotherapy group. These results demonstrate the potential of ADCs to improve treatment outcomes in cancer patients. These results are significantly better than those achieved with traditional chemotherapy or HER2-targeted therapies alone.
The use of ADCs can increase efficacy in cancer therapy because they can selectively target cancer cells while sparing healthy cells. This is because the monoclonal antibodies in ADCs are designed to recognize and bind to specific antigens that are overexpressed on the surface of cancer cells. Once the ADC binds to the cancer cell, the chemotherapy drug is released and kills the cancer cell. This targeted approach can reduce the side effects associated with traditional chemotherapy, which can damage healthy cells as well as cancer cells.
The use of ADCs can increase efficacy in cancer therapy because they can selectively target cancer cells while sparing healthy cells. This is because the monoclonal antibodies in ADCs are designed to recognize and bind to specific antigens that are overexpressed on the surface of cancer cells. Once the ADC binds to the cancer cell, the chemotherapy drug is released and kills the cancer cell. This targeted approach can reduce the side effects associated with traditional chemotherapy, which can damage healthy cells as well as cancer cells.
Minimizing systematic drug distribution
ADC technology can minimize systemic drug distribution in cancer treatment by selectively delivering cytotoxic drugs to tumor cells while sparing healthy cells. The large size of ADCs minimizes their distribution into metabolizing and healthy tissues, and ADCs provide a selective targeting mechanism for cytotoxic drugs, improving the therapeutic index in clinical practice. The primary purpose of ADCs is to increase the efficacy of anticancer medications by minimizing systemic drug distribution and targeting specific cells, and ADCs successfully minimize the systemic toxicity of chemotherapy and provide novel therapeutic alternatives for diseases with poor prognoses and few treatment options.
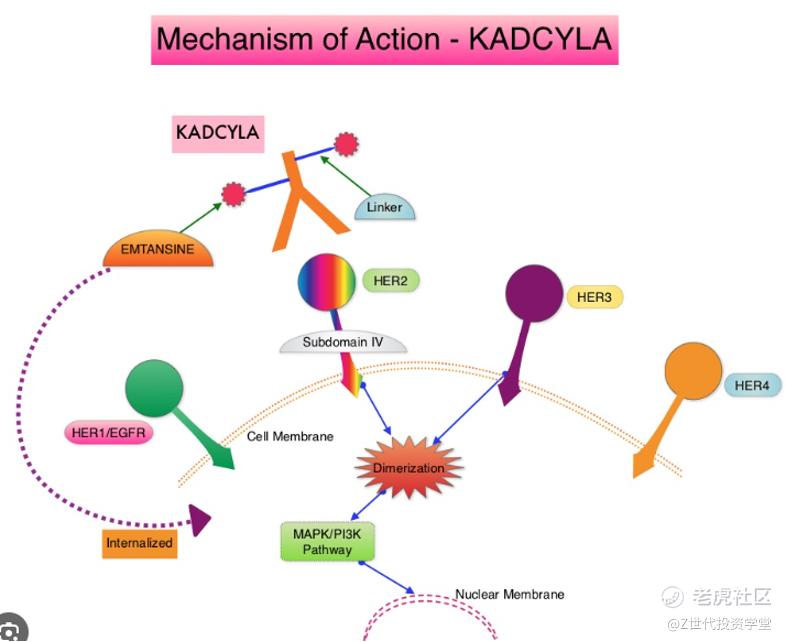
Advanced drug delivery systems l provides a targeted approach for delivering cytotoxic chemotherapy to tumors, while limiting effects on healthy cells. For example, ado-trastuzumab emtansine (Kadcyla) is an ADC used to treat HER2-positive breast cancer. It consists of the monoclonal antibody trastuzumab linked to the chemotherapy drug DM1 via a stable linker. The trastuzumab selectively binds to HER2 receptors on breast cancer cells.
Once bound, the entire ADC complex is internalized into the cell. Inside the cancer cell, enzymes cleave the linker, releasing DM1 to cause cell death. This targeted release mechanism allows Kadcyla to achieve powerful antitumor activity while bypassing healthy cells that do not overexpress HER2. As a result, it can treat breast cancer more effectively at a lower systemic dose of DM1, reducing side effects compared to traditional chemotherapy. Kadcyla's antibody component also serves to repeatedly transport DM1 back inside tumor cells, ensuring a lasting response with fewer treatment cycles than standard therapy alone.
Alternative therapy for previously unmet medical needs
For many years, certain types and subtypes of breast cancer presented formidable challenges due to resistance to existing therapies or lack of treatment options. However, recently approved ADCs have begun to fulfill significant unmet medical needs in these hard-to-treat settings.
One area where ADCs have been transformative is in treating HER2-positive breast cancer. This aggressive cancer subtype accounts for around 20% of cases but was difficult to manage for many years. Traditional chemotherapy could control the disease for a time but did not provide a lasting benefit and toxicity was a major concern. The approval of ado-trastuzumab emtansine (Kadcyla) in 2013 changed this. As an ADC, Kadcyla delivers the potent chemotherapy agent DM1 directly to HER2-expressing breast cancer cells via the targeting antibody trastuzumab. In clinical trials, Kadcyla demonstrated a 33% reduced risk of disease progression or death compared to lapatinib plus capecitabine in patients who recurred after prior treatment. Its targeted mechanism allows effective control of HER2-positive breast cancer at lower system doses than standard chemotherapy alone, fulfilling an important unmet need.
For patients with triple negative breast cancer, which lacks hormone receptors and HER2 expression, limited effective therapies also existed. This cancer is often resistant to traditional chemotherapy. However, new ADCs have shown promise in trials. Sacituzumab govitecan (Gilead) for instance, delivers the topoisomerase inhibitor SN-38 to cancer cells expressing the target Trop-2. In a phase 3 trial, sacituzumab govitecan reduced the risk of death for patients with metastatic triple negative breast cancer by 21% compared to single-agent chemotherapy. Its targeted profile may overcome resistance and fulfill an unmet need for this difficult-to-treat patient subgroup.
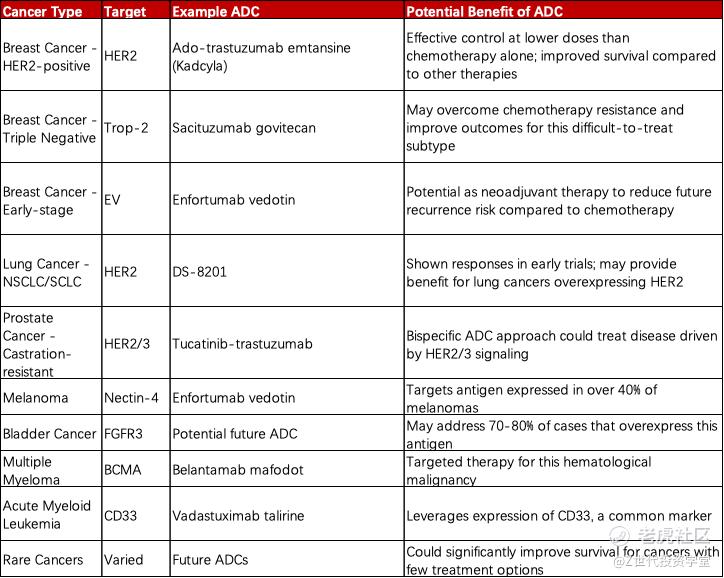
While ADCs have made great strides in treating cancers like HER2-positive breast cancer, their potential is not limited to just a few tumor types. Researchers are actively exploring the use of ADCs across multiple other solid and hematological malignancies where this approach could provide major benefits. Lung cancers, the leading cause of cancer death worldwide, express antigens ripe for ADC targeting like HER2; early results with DS-8201 have been promising. Prostate cancer driven by HER2/3 signaling may respond to novel bispecific ADCs as well. Melanoma, bladder cancer, and other solid tumors overexpressing proteins such as Nectin-4 and FGFR3 are also candidates. In hematologic cancers, BCMA and CD33 ADCs have demonstrated activity in diseases as prevalent as multiple myeloma and acute myeloid leukemia. Even rarer cancers, where survival rates are notoriously low due to lack of options, could see improved outcomes from precisely-targeted ADCs. Neoadjuvant and adjuvant settings pre- and post-surgery are also under exploration, with the goal of reducing risk of future recurrence or metastasis across cancer types. Continued advancements in ADC technology spearhead investigations into these diverse indications that may transform treatment.
修改于 2024-04-01 22:39
免责声明:上述内容仅代表发帖人个人观点,不构成本平台的任何投资建议。