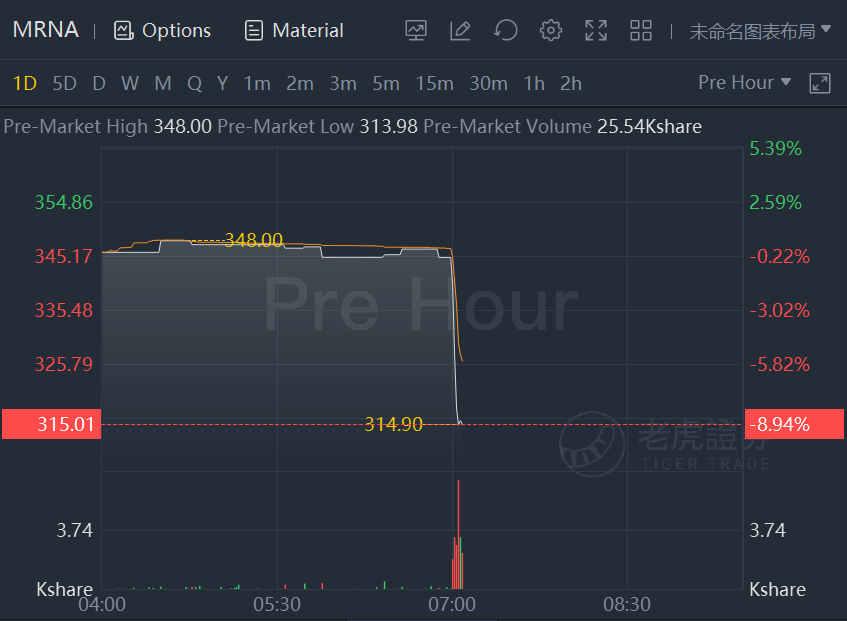
Moderna stock plunged 9% after it cut its Covid-19 vaccine sales forecast for the year and missed earnings and revenue expectations for the third quarter.
- Moderna Q3 revenue $4.969 bln vs. $157 mln a year ago; FactSet consensus $6.200 bln;
- Moderna Q3 EPS $7.70 vs loss 59 cents a share a year ago; FactSet consensus $9.09;
Moderna, Inc. today reported financial results and provided business updates for the third quarter of fiscal year 2021.
Q3 total revenue of$5.0 billion, net income of$3.3 billionand diluted EPS of$7.70
U.S.FDA granted Priority Review to the Biologics License Application for Moderna’s COVID-19 vaccine
Interim data from Phase 2/3 KidCOVE study of mRNA-1273 in children ages 6 to under 12 years shows vaccine efficacy of 100% two weeks after first dose of mRNA-1273 at 50 µg dose level
First participants dosed in Phase 3 study of cytomegalovirus (CMV) vaccine candidate (mRNA-1647)
Introducing inhaled pulmonary therapeutics modality:Vertex andModernacystic fibrosis mRNA therapeutic (VXc-522) IND-enabling first-in-human studies ongoing
Company continues to scale with 37 programs in development, including 21 in ongoing clinical studies
Updates and recent progress include:
COVID-19 Vaccine Development
- Moderna COVID-19 Vaccine (SpikevaxTM): Received Emergency Use Authorization (EUA) from theU.S. Food and Drug Administration(U.S.FDA), and approvals by theEuropean CommissionandSwissmedicfor a booster dose of the Moderna COVID-19 vaccine at the 50 µg dose level
- U.S.FDA granted Priority Review to the Biologics License Application (BLA) for the Moderna COVID-19 vaccine
- New data from Phase 2/3 KidCOVE study of mRNA-1273 in children ages 6 to under 12 years shows vaccine efficacy of 100% two weeks after first dose of mRNA-1273 at 50 µg dose level, using the Phase 3 COVE study primary case definition for COVID-19
- The Phase 1 study of next-generation vaccine candidate against COVID-19 (mRNA-1283) is fully enrolled;Modernaexpects to begin Phase 2 study of mRNA-1283 soon; mRNA-1283 is being developed as a potential refrigerator-stable mRNA vaccine
Respiratory Vaccines
- Phase 1 portion of the Phase 1/2 study of quadrivalent seasonal flu vaccine candidate (mRNA-1010) fully enrolled, preparations for Phase 2 portion of the study are ongoing
- Pivotal Phase 2/3 study of respiratory syncytial virus (RSV) vaccine candidate (mRNA-1345) in older adults expected to begin in 2021; study expected to enroll approximately 34,000 participants, subject to agreement with regulatory authorities
- New combination respiratory vaccines: Moderna COVID-19 vaccine + flu vaccine candidate (mRNA-1073) and pediatric RSV + hMPV vaccine candidate (mRNA-1365)
Latent Vaccines
- First participants dosed in Phase 3 study of cytomegalovirus (CMV) vaccine candidate (mRNA-1647)
- Phase 1 study of EBV vaccine candidate (mRNA-1189)expected to start soon
- New EBV therapeutic vaccine candidate (mRNA-1195)
Therapeutics
- Phase 2 randomized, placebo-controlled study of personalized cancer vaccine (PCV) (mRNA-4157) in combination with Merck’s pembrolizumab (KEYTRUDA®), compared to pembrolizumab alone, for the adjuvant treatment of high-risk resected melanoma is fully enrolled; data readout expected in the fourth quarter of 2022
- Enrollment of the first cohort in Propionic Acidemia candidate (mRNA-3927) Phase 1/2Paramountstudy is complete
- First patient dosed in Phase 1 study of Methylmalonic Acidemia (MMA) candidate (mRNA-3705)
- Investigational New Drug application (IND) open and Orphan Drug Designation granted byU.S.FDA for GSD1a program (mRNA-3745)
- Providing investigational mRNA Crigler-Najjar Syndrome Type 1 (CN-1) therapy (mRNA-3351) toInstitute for Life Changing Medicines(ILCM) free of charge; CN-1 is an ultra-rare disease
- Introducing inhaled pulmonary therapeutics modality; IND-enabling first-in-human studies of Vertex andModernamRNA cystic fibrosis (CF) therapeutic (VXc-522) are ongoing in new pulmonary modality
Moderna continues to scale, now with 37 programs in development across 34 development candidates1, including 21 in ongoing clinical studies. The Company’s updated pipeline can be found atwww.modernatx.com/pipeline.Modernaand collaborators have published nearly 100 peer reviewed manuscripts.
2021 Updated Financial Framework
- For Expected Delivery in Fiscal Year (FY) 2021:Expected to realize product sales for FY 2021 between$15 billionand$18 billion.
- Vaccine Dose Deliveries for FY 2021:The Company expects deliveries of its COVID-19 vaccine in FY 2021 to be between 700 million and 800 million doses at the 100 µg dose level. Key variables impacting output include longer delivery lead times for international shipments and exports that may shift deliveries to early 2022, temporary impact from expansion of fill/finish capacity and ramp up of product release to market.
- Cost of Sales:Cost of sales as percentage of product sales are expected to be between 16-17% for FY 2021.
- 2021 Research & Development (R&D) and Selling, General & Administrative (SG&A) Expenses:Continue to expect quarter over quarter cost increases in R&D and SG&A expenses during 2021 as commercial and research and development activities and expenses ramp up.
- Tax Rate:The Company now expects the effective tax rate for 2021 to be in the high single digit range as a result of the forecasted global sales mix and utilization of the accumulated net operating loss carry-forward of$2.3 billion.
- Capital Expenditures:Expect approximately$0.4 billionof capital investments for 2021.
- Share Repurchase Program:The Board of Directors has authorized a share repurchase program of up to$1 billionover a two-year period to return excess capital to shareholders. No shares were repurchased through the end of the third quarter.
2022 Revenue Drivers
They expect several dynamics will drive 2022 revenues:
- APAs Signed:The Company has signed approximately$17 billionof advance purchase agreements (APAs) for delivery in 2022.
- APAs with Options:The Company anticipates the exercise of options under 2022 APAs of up to$3 billion.
- U.S.Fall 2022 Booster Market:Subject to receipt of a BLA or sBLA for boosters prior to the fall booster season, the Company anticipates commercial booster market sales could be up to$2 billion.
Based on these three revenue drivers, the Company believes 2022 sales could be in the range of$17 billionto$22 billion. The Company continues to have discussions for 2022 APAs with governments and international organizations, including COVAX, thePan American Health Organization(PAHO) and theAfrican Union.
精彩评论